What is Ozone?
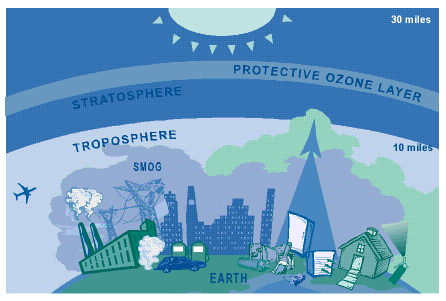
Ozone is a natural gas that is found in two
different layers of the atmosphere. In the layer around the Earth's surface,
otherwise known as the troposphere, bad ozone dirties the air and helps make
smog. The troposphere extends up to the stratosphere layer, where good ozone
protects life on Earth by absorbing some of the sun's UV rays. Stratospheric
ozone is found most often between six to 30 miles above the Earth's surface.
What is the ozone layer?
The Earth is wrapped in a blanket of air called the 'atmosphere', which is made up of several layers. About 19-30 kilometres above the Earth is a layer of gas called ozone, which is a form of oxygen. Ozone is produced naturally in the atmosphere.
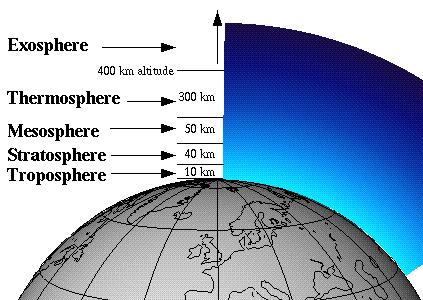

Why do we have Ozone Layer?
The ozone layer is very important because it stops too many of the sun's 'ultra-violet rays' (UV rays) getting through to the Earth - these are the rays that cause our skin to tan. Too much UV can cause skin cancer and will also harm all plants and animals. Life on Earth could not exist without the protective shield of the ozone layer.
What is Stratospheric Ozone?
 Ozone is a natural gas that is found in two different layers of the atmosphere.
One layer, called the troposphere, is at the Earth's surface where we live.
Ozone in the troposphere is "bad" because it dirties the air and helps make smog, which is unhealthful to breathe.
The other layer, called the stratosphere, is miles above the Earth's surface. Ozone in the stratosphere is "good" because it protects life on Earth by absorbing some of the sun's harmful UV rays.
Stratospheric ozone is found most often between six and 30 miles above the Earth's surface.
Ozone is a natural gas that is found in two different layers of the atmosphere.
One layer, called the troposphere, is at the Earth's surface where we live.
Ozone in the troposphere is "bad" because it dirties the air and helps make smog, which is unhealthful to breathe.
The other layer, called the stratosphere, is miles above the Earth's surface. Ozone in the stratosphere is "good" because it protects life on Earth by absorbing some of the sun's harmful UV rays.
Stratospheric ozone is found most often between six and 30 miles above the Earth's surface.
What is Ozone Depletion?
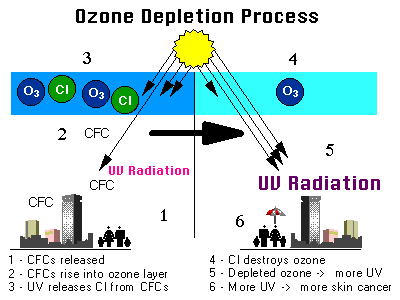 Recently, chlorofluorocarbons (CFCs) were used a lot in industry and elsewhere to keep things cold and to make foam and soaps.
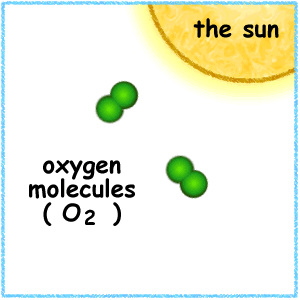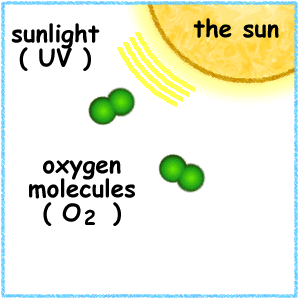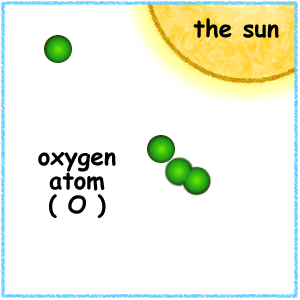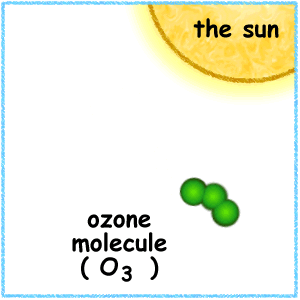
Strong winds carry CFCs up into the stratosphere where UV radiation breaks them apart, releasing chlorine atoms.
Each chlorine atom can attack and break apart (destroy) as many as 100,000 ozone molecules during the time it is in the stratosphere.
The chlorine from CFCs reduces (depletes) the amount of ozone in the stratosphere.
Other ozone-eating chemicals are pesticides such as methyl bromide, halons used in fire extinguishers, and methyl chloroform used in businesses.
Recently, chlorofluorocarbons (CFCs) were used a lot in industry and elsewhere to keep things cold and to make foam and soaps.
Strong winds carry CFCs up into the stratosphere where UV radiation breaks them apart, releasing chlorine atoms.
Each chlorine atom can attack and break apart (destroy) as many as 100,000 ozone molecules during the time it is in the stratosphere.
The chlorine from CFCs reduces (depletes) the amount of ozone in the stratosphere.
Other ozone-eating chemicals are pesticides such as methyl bromide, halons used in fire extinguishers, and methyl chloroform used in businesses.
How Ozone Depletion Affects UV Levels?
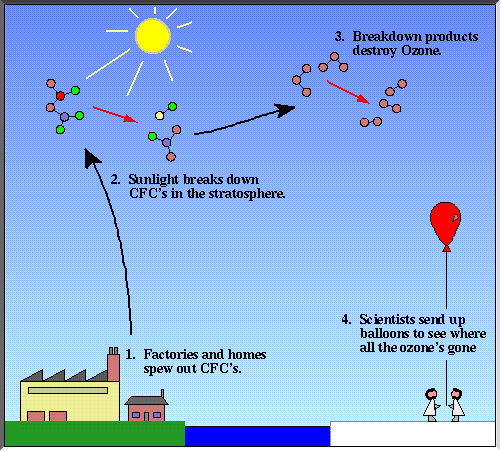 Scientists predict that ozone depletion should peak around year 2010.
As world-wide controls reduce the release of CFCs and other ozone-eating substances, nature will repair the ozone layer.
By year 2065 stratospheric ozone should return to the amount present in 1980. Until then, we can expect higher levels of UV radiation at the Earth's surface.
We need to take care to avoid the bad health effects that could result from too much UV radiation.
Scientists predict that ozone depletion should peak around year 2010.
As world-wide controls reduce the release of CFCs and other ozone-eating substances, nature will repair the ozone layer.
By year 2065 stratospheric ozone should return to the amount present in 1980. Until then, we can expect higher levels of UV radiation at the Earth's surface.
We need to take care to avoid the bad health effects that could result from too much UV radiation.
What is Ozone Hole?
 Every spring, a hole as big as the USA develops in the ozone layer over Antarctica, in the South Pole. A smaller hole develops each year over the Arctic, at the North Pole. And there are signs that the ozone layer is getting thinner all over the planet.
Every spring, a hole as big as the USA develops in the ozone layer over Antarctica, in the South Pole. A smaller hole develops each year over the Arctic, at the North Pole. And there are signs that the ozone layer is getting thinner all over the planet.
 Scientists have discovered that the ozone hole over Antarctica started in 1979, and that the ozone layer generally started to get thin in the early 1980s.
Scientists have discovered that the ozone hole over Antarctica started in 1979, and that the ozone layer generally started to get thin in the early 1980s.
The loss of the ozone layer occurs when more ozone is being destroyed than nature is creating.
What causes the Ozone Hole?
 One group of gases is particularly likely to damage the ozone layer. These gases are called CFCs, Chloro-Fluoro-Carbons.
One group of gases is particularly likely to damage the ozone layer. These gases are called CFCs, Chloro-Fluoro-Carbons.
 CFCs are used in some spray cans to force the contents out of the can.
CFCs are used in some spray cans to force the contents out of the can.
 They are also used in refrigerators, air conditioning systems and some fire extinguishers. They are used because they are not poisonous and do not catch fire.
They are also used in refrigerators, air conditioning systems and some fire extinguishers. They are used because they are not poisonous and do not catch fire.
Most countries have now stopped using new CFCs that can be released into the atmosphere, but many scientists believe we must stop using old ones as well.
THE OZONE HOLE AND OUR HEALTH
 The ozone layer is like a sunscreen, and a thinning of it would mean that more ultra-violet rays would be reaching us.
The ozone layer is like a sunscreen, and a thinning of it would mean that more ultra-violet rays would be reaching us.
 Too many UV rays would cause more sunburn, and because sunburn causes skin cancer, this too would increase deaths.
Too many UV rays would cause more sunburn, and because sunburn causes skin cancer, this too would increase deaths.
These UV rays are also dangerous for our eyes and could cause an increase in people becoming blind. That is why sun cream and sunglasses are very important.
THE OZONE HOLE ON ANIMALS AND PLANTS
 UV rays can go through water and end up killing small water animals or plants, called 'plankton' which form the base of the food chain in oceans and seas. Whales and other fishes have plankton as their main food, and if plankton die because of these UV rays, whales will start dying too, because they will not have anything to eat.
UV rays can go through water and end up killing small water animals or plants, called 'plankton' which form the base of the food chain in oceans and seas. Whales and other fishes have plankton as their main food, and if plankton die because of these UV rays, whales will start dying too, because they will not have anything to eat.
 Large amounts of UV rays could damage all green plants. If the ozone layer keeps getting thinner, there could be fewer and fewer plants on Earth, then there would be less food in the whole world.
Large amounts of UV rays could damage all green plants. If the ozone layer keeps getting thinner, there could be fewer and fewer plants on Earth, then there would be less food in the whole world.
THE TWO-FACED OZONE GAS
 Ozone found between 19 and 30 kilometres high in the atmosphere is one of the reasons why we are alive on Earth.
Ozone found between 19 and 30 kilometres high in the atmosphere is one of the reasons why we are alive on Earth.
 But when the gas ozone is found lower down where we can breathe it in, it becomes very dangerous for our health. This ozone is caused by a reaction between air pollution and sunlight and can cause modern-day smog. This is different to the smog that formed in the early 20th century from smoke and fog.
But when the gas ozone is found lower down where we can breathe it in, it becomes very dangerous for our health. This ozone is caused by a reaction between air pollution and sunlight and can cause modern-day smog. This is different to the smog that formed in the early 20th century from smoke and fog.